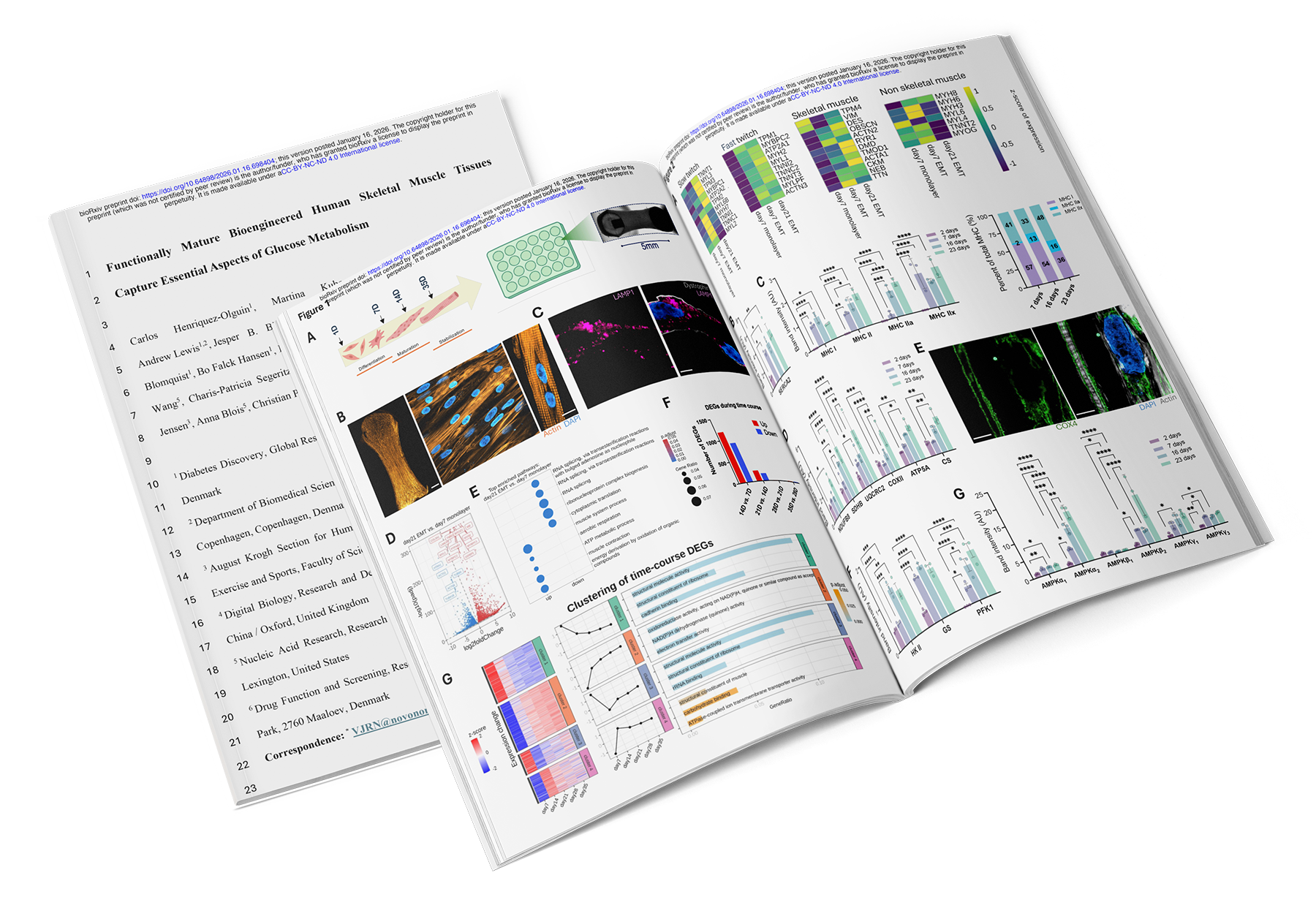
Originally Published in: BioRxiv (January 2026) (Download the Preprint)
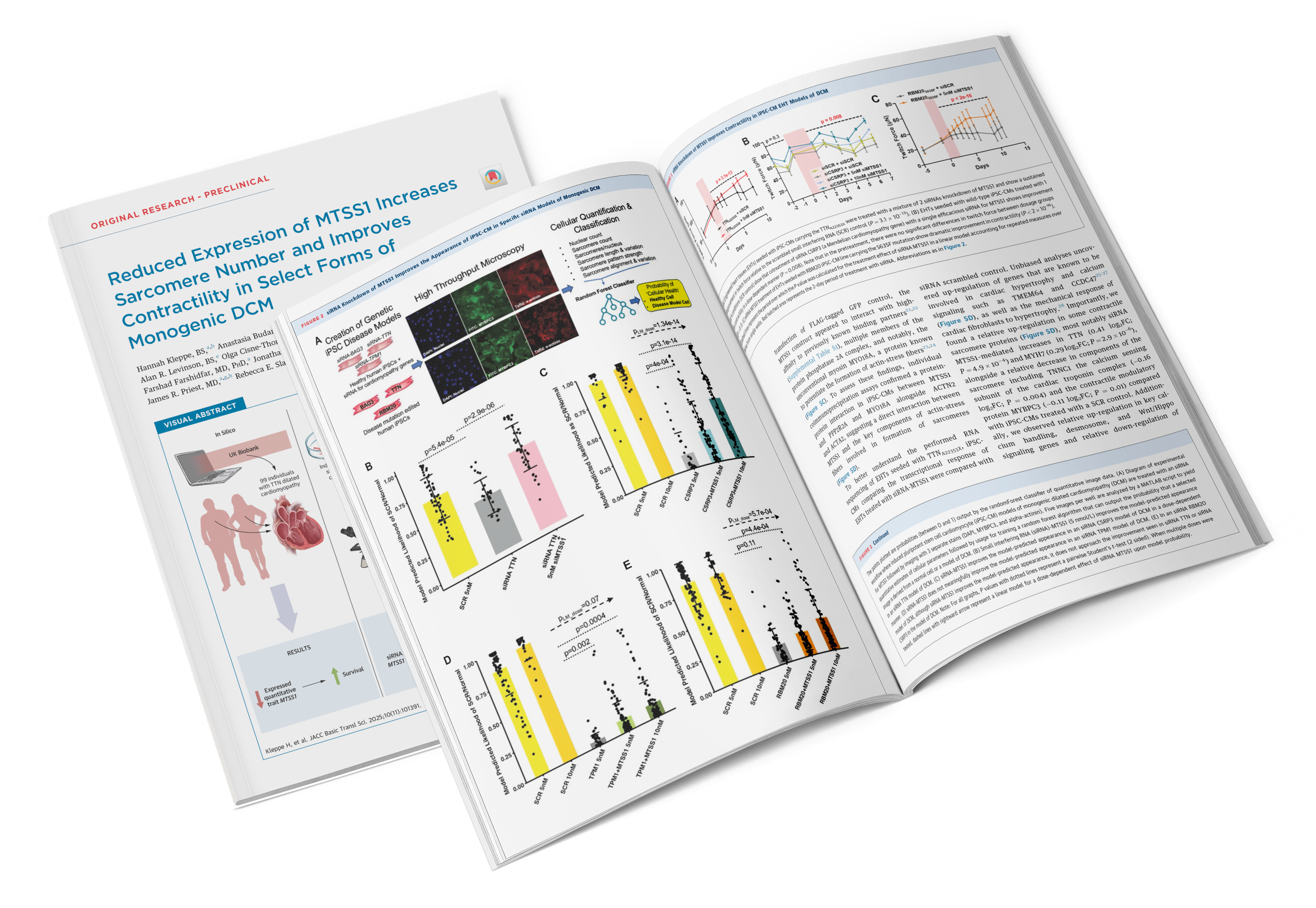
Read MoreOriginally Published in: JACC: Basic to Translational Science (December 2025) (Download the Publication)
Read MoreOriginally Published in: Journal of Tissue Engineering (September 2025) (Download the Publication)
Read MoreOriginally Published in: bioRvix (August 2025) [Link to Full Publication]
Read MoreOriginally Published in: bioRxiv (June 2025) (Link to Original Posting)
Read MoreOriginally Published in: Communications Biology (May 2025) [Link to Full Publication]
Read MoreOriginally Published in: Nature Communications (February 2025) (Link to Original Posting)
Read MoreOriginally Published in: bioRxiv (December 2025) [Download the Publication]
Read MoreOriginally Published in: Advanced Science (January 2025) [Read the Publication]
Read MoreOriginally Published in: Science Direct (January 2025) (Link to Original Posting)
Read MoreData from Tenaya Therapeutics (2022)
Read MoreData from Entrada Therapeutics (2024)
Read MoreOriginally Published in: Communications Medicine (March 2024) (Link to Paper)
Read MoreOriginally Published in: Biomaterials Science (2023) (Link to Paper)
Read MoreOriginally Published in: JoVE Journal (2023) (Link to Paper)
Read MoreOriginally Published in: eLife (2021) (Link to Paper)
Read MoreOriginally Published in: Sage Journals (2022) (Link to Paper)
Read MoreOriginally Published in: Nature Scientific Reports (2022) (Link to Paper)
Read MoreOriginally Published in: Archives of Physical Medicine and Rehabilitation - V103, Issue 3 (2022) (Link to Paper)
Read More