Originally Published in: Experimental Eye Research (2020) (Link to Paper)
Read MoreOriginally Published in: Nature Communications (2019) (Link to Paper)
Read MoreAlec S. T. Smith, Eunpyo Choi, Kevin Gray, Jesse Macadangdang, Eun Hyun Ahn, Elisa C. Clark, Michael A. Laflamme, Joseph C. Wu, Charles E. Murry, Leslie Tung, Deok-Ho Kim* Nano Lett. 2020, vol. 20, no. 3, pp. 1561-1570, Publication Date: December 17, 2019 (Link to Paper)
Read MoreKshitiz, Afzal, J., Maziarz, J.D. et al. Nat Ecol Evol 3, pp. 1743–1753, Publication Date: 2019 (Link to Paper)
Read MoreOriginally Published in: Nature Communications (2019) (Link to Paper)
Read MoreOriginally Published in: Advanced Science (2019) (Link to Paper)
Read MoreBong Hwan Sung, Roxanne Pelletier, Alissa M. Weaver, bioRxiv 577346, Publication Date: 2019 (Link to Paper)
Read MoreOriginally Published in: Cell Reports (2018) (Link to Paper)
Read MoreKim, D., Ewald, A.J., Park, J. et al. Sci Rep 8, 14210, Publication Date: 2018 (Link to Paper)
Read MoreJonathan H. Tsui et al. J. Mater. Chem. B, 6, 7185-7196, Publication Date: 2018 (Link To Paper)
Read MoreJoong-Hyun Kim et al. J Periodontal Implant Sci. , 47(6): 402–415, Publication Date: December 2017 (Link To Paper)
Read MoreJonathan H. Tsui et al. ACS Nano, 11, 12, 11954-11968, Publication Date: November 20, 2017 (Link To Paper)
Read MoreLi, Linfeng & LaBarbera, D.V. 10.1016/B978-0-12-409547-2.12329-7., Publication Date: 2016 (Link To Paper)
Read MoreRay, A., Lee, O., Win, Z. et al. Nat Commun 8, 14923, Publication Date: 2017 (Link To Paper)
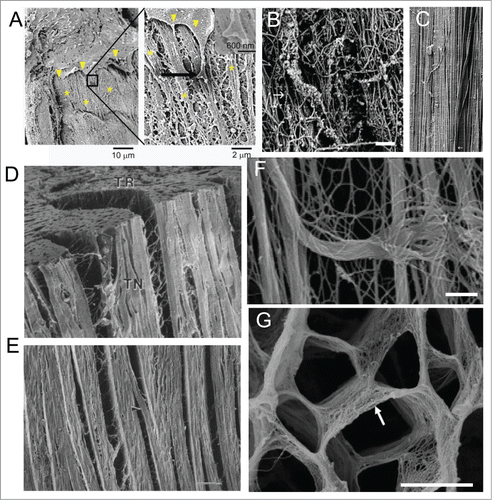
Read MoreSource: Nisa P. Williams, Marcus Rhodehamel, Calysta Yan, Alec S.T. Smith, Alex Jiao, Charles E. Murry, Marta Scatena, Deok-Ho Kim, Engineering anisotropic 3D tubular tissues with flexible thermoresponsive nanofabricated substrates, Biomaterials, Volume 240, 2020, 119856, ISSN 0142-9612.
(Link to Paper)
Kshitiz, Junaid Afzal, Sang-Yeob Kim & Deok-Ho Kim, Cell Adhesion & Migration, 9:4, 300-307, DOI, Publication Date: 2015 (Link To Paper)
Read MoreHyeona Jeon et al. ACS Appl. Mater. Interfaces 2015, 7, 8, 4525-4532, Publication Date: February 6, 2015 (Link To Paper)
Read MoreCameron L. Nemeth et al. Tissue Engineering Part A VOL. 20, NO. 21-22, Publication Date: 25 Jun 2014 (Link To Paper)
Read MorePeter Kim et al. Biofabrication, Volume 6, Number 2, Date Published: 10 April 2014 (Link To Paper)
Read More